Background: Luspatercept is the first erythropoiesis-stimulating agent approved for transfusion-dependent β-thalassemia (TDT). In the BELIEVE trial, more than 70% of the patients had a hematological response defined as a reduction of ≥33% of transfusion burden in any 12-week interval. In our experience, some patients have a clinical benefit even if a response as defined by the clinical trial is not reached. Indeed, several aspects remain to be addressed, including its specific effects on erythropoiesis. Fetal hemoglobin (HbF) has been hypothesized as a potential marker in response evaluation. However, no results have been published, excluding an abstract presentation by Cappellini MD et al. at the European Hematology Association meeting in 2019, showing a greater HbF increase in responders vs. non-responders.
Aim: This study aims to evaluate the effect of luspatercept on erythropoiesis and the hemoglobin (Hb) fractions in patients with β-TDT treated with luspatercept in a real-life setting at our Center in Milan, Italy.
Methods: Inclusion criteria: age ≥18 years, β-TDT eligible for treatment with luspatercept, and signed informed consent. Exclusion criteria: unwilling to participate in the study. We assessed hematological parameters, Hb fractions, and erythropoiesis markers at baseline and over the treatment period of up to 48 weeks (Dose16). We also extracted mRNA from reticulocytes (RET) from peripheral blood at fixed doses and analyzed the expression of a panel of genes involved in erythropoiesis.
We evaluated hematological variables dividing the patients into three groups of responses defined:
Full responder (FR): reduction of transfusion burden ≥33% and ≥2 units of blood in any 12-week interval
Intermediate responder (IR): patients with a reduction in transfusion burden ≥25% and <33% in any 12-week interval
Non-responder (NR): reduction of the transfusion burden <25%.
Statistical analysis at baseline was performed using Kruskal-Wallis test. We fitted linear random intercept regression models to evaluate time trends of hematological variables.
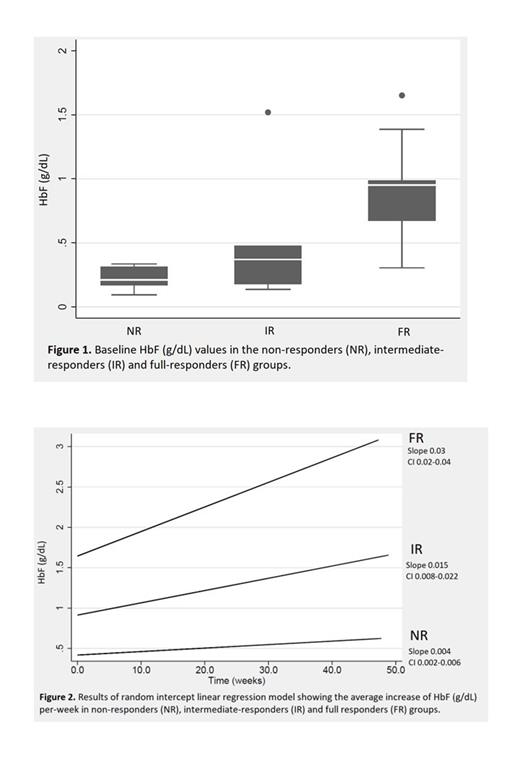
Results: Thirty-seven patients have been enrolled in the study. 3/37 have been excluded from the analysis because they developed adverse events leading to drug interruption before completing 12 weeks of treatment. We herein present the data of the 29/34 subjects who reached at least 12 weeks of treatment as of July 20, 2023. The median age was 41 yrs (range 19-63 yrs), 15 were females (52%). 8/29 (28%) had a β0/β0 genotype; 12/29 (41%) patients underwent splenectomy. Twelve patients had a FR, 6 an IR, and 11 were NR. We found that the baseline median absolute value of HbF (g/dL) was different in the three groups (NR: 0.21, range 0.09-0.34; IR: 0.37, range 0.14-1.52; FR: 0.95, range 0.31-1.65, p=0.009) (Figure 1). In all subjects, HbF increased over time. However, the increase was different in the three groups (p-interaction: 0.0001). In the linear regression model (Figure 2), the HbF absolute increase per week was 0.004 g/dL (95% confidence interval (CI): 0.002-0.006) among NR, 0.015 (CI: 0.008-0.022) in the IR group and 0.03 g/dL (CI: 0.02-0.04) in the FR group. EPO baseline value was similar in the three groups, but unexpectedly we observed different increases over time (p-interaction: 0.0001). In the IR and FR groups, the regression slopes were 6.66 U/L (CI: 3.11-10-21) and 9.42 U/L (CI: 5.53-13.31) per week, respectively. In the NR group, there was no EPO increase. Interestingly, the baseline value of erythroblasts (EB) was different between the three groups, with FR having the highest value. EB increased in both IR and FR, with the IR group displaying a steeper increase and finally reaching the FR group. Preliminary results on gene expression in RET showed an upregulation of GATA-1 and its target genes involved in erythroid differentiation and heme biosynthesis, as shown in the murine model (Martinez PA et al. 2019), irrespective of the response group.
Conclusions: Our findings identify baseline HbF as a possible predictor of response. Also, the HbF trend could represent a marker of response to luspatercept in β-TDT patients. Furthermore, HbF can be useful in identifying patients in the IR group with the best chance of becoming responders in long-term treatment. Our preliminary results also suggest that luspatercept acts by enhancing erythropoiesis and leads to an increase in EPO levels and EB. Studies on more patients are needed to confirm these findings.
Disclosures
Motta:Amicus Therapeutics: Consultancy; Sanofi: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bristol-Myers Squibb: Consultancy, Speakers Bureau.